Fuel cells are revolutionizing the way we think about energy. From powering homes to fuelling cars, they are a cleaner and more sustainable alternative for fossil fuels.
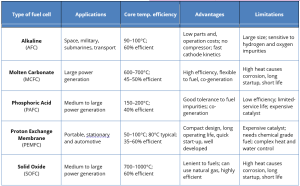
Based on the kind of electrolyte, there are three main types of fuel cells. That classification determines what kind of electrochemical reactions takes place in the cell, what types of catalyst are required, temperature range for operation of the cell, type of fuel needed etc .
Here is a closer look at five primary fuel cell types, how they operate, and where you can find them in the real world.
What are fuel cells and How Do they Work?
Fuel cells (FCs) are electrochemical devices that convert the chemical energy of fuels, commonly hydrogen, directly into electricity. They emit very little pollution compared to a combustion engine, so they are an environmentally friendly source of energy.
Fuel cells quickly switch energy needs flow now because of their versatility, efficiency, and low environmental impact. They are especially important for supporting efforts like the decarbonization of the economy where we no longer depend on fossil fuels.
Types of Fuel Cells
PEM fuel cell (proton exchange membrane )
PEM fuel cells use a solid polymer as an electrolyte and porous carbon electrodes containing a platinum or platinum alloy catalyst. PEMFC have a low operational temperature (~ 80 °C | 176 °F) Their low operating temperature enables quick starts (lower warm-up time), produces less stress on parts, and provides better longevity. PEM fuel cells are mainly utilized for transport applications and few stationary applications.
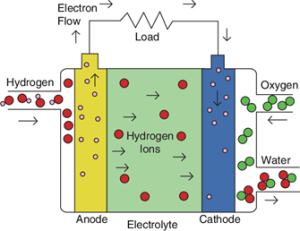
How It Works
At the anode, hydrogen is what gets oxidized to generate paramagnetic protons and electrons. As protons make their way through the membrane, electrons flow along an external circuit to produce electricity.
Applications of PEMFC
- Portable power systems
- Fuel cell vehicles
- Home backup power
Benefits:
Very short startup time, small form factor, and low running temperatures.
Drawbacks:
Catalysts are expensive and sensitive to fuel contamination
Solid Oxide Fuel Cell (SOFC)
SOFCs use a solid ceramic electrolyte with operating temperatures of 600-1000 °C. The electrolyte is a hard, non-porous ceramic compound. Fuel to electricity conversion is approximately 60% efficient with SOFCs. In cogeneration applications where waste heat is recovered and used , the total fuel use efficiencies might exceed 85%.
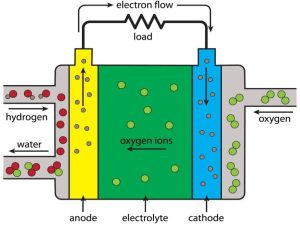
Working Principle
The oxygen ions pass through the ceramic electrolyte, where they react with hydrogen producing electricity, water and heat.
Key Uses of SOFC
- Industrial plant power generation
- Cogeneration
Advantages
Great efficiency, and can use hydrocarbons
Disadvantages
SOFCs work in extremely high temperatures as much as 1,000°C (1,830°F). which degrades the materials over time.
Alkaline Fuel Cell (AFC)
Alkaline fuel cells (AFCs) employ a potassium hydroxide electrolyte. As one of the earlier developed fuel cell technologies, AFCs were the first to be used in the US space program to generate electrical energy and water on board vehicles. These are fuel cells similar to regular PEM fuel cells, only in place of an acid membrane, an alkaline membrane is used. The reason why AFCs score high on performance is due to the rate of the electro-chemical reactions that take place within the cell. They have also shown 60% efficiency for space applications.
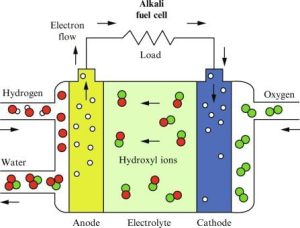
Functionality and Mechanism
Alkaline fuel cells use a solution of potassium hydroxide in water as the electrolyte and can use a variety of non-precious metals as a catalyst at the anode and cathode. The hydrogen and the oxygen react in an alkaline medium producing electricity, water, and heat .
Common Applications
- Space missions (Apollo program, etc.)
- Backup power
Advantages:
It is very efficient and reliable.
Disadvantages :
Susceptible to CO2 contamination.
Phosphoric Acid Fuel Cell (PAFC)
Phosphoric acid fuel cells (PAFCs) operate at medium temperature (150–200°C) and use phosphoric acid. In phosphoric acid fuel cells (PAFCs), liquid phosphoric acid is the electrolyte, contained within a Teflon-bonded silicon carbide matrix, porous carbon electrodes with a platinum catalyst are used for both the anode and the cathode.
So the PAFC is called a “first generation” fuel cell. It is one of the most mature cell types and the first to be used commercially. Stationary use is the most common for this fuel cell, but some PAFC have been used in large vehicles like city bubbles.
The efficiency of PAFC is limited to a little bit more than that of combustion-based power plants, these operate typically at about 33% efficiency. By weight and volume, PAFCs are also weaker than the other types of fuel cells. Consequently, these fuel cells tend to be bulky and heavy. PAFCs are also expensive. They also use far more platinum catalyst than other types of fuel cells, which drives up the price.
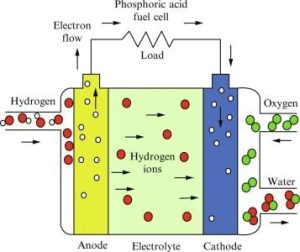
How It Operates
Hydrogen in the presence of a catalyst reacts with oxygen to generate electricity and water.
Primary Uses
- Power and heat for hospitals and hotels
- Small-scale power plants
Advantages:
Very tolerant of fuel contaminants.
Disadvantages:
Size and efficiency as they are larger, there are smaller ones but not in every case.
Molten Carbonate Fuel Cell (MCFC)
Molten carbonate fuel cells (MCFC) are high temperature (600-7000), using a molten carbonate salt mixture as the electrolyte. MCFCs are being developed for the electrical utility, industrial, and military applications using natural gas or coal-based power plants.
MCFCs utilize a molten carbonate salt mixture dispersed in a porous, chemically inert ceramic lithium aluminum oxide matrix. These allow for non-precious metals to be used as anode and cathode catalysts, which is a cost saving option due to operating temperatures of 650°C (Approximately 1,200°F).
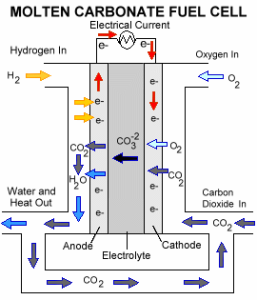
Basic Working Principle
The carbonate ions migrate through the electrolyte to enable the electrochemical reaction of hydrogen with oxygen.
Industrial Applications
- Power generation with large-scale utility
- Carbon capture systems
Pros :
High efficiency, can use multiple fuels
Cons :
Issues with corrosion and durability.
How Do They Compare against Different Fuel Cells
Efficiency Levels : SOFCs and MCFCs are the most efficient, while PEMFCs operate at a lower efficiency level but are quicker to startup.
Working conditions : PEMFCs working in lower temperatures, but high temperatures (HT) are necessary for SOFC and MCFC.
Applications Best For : vehicles (PEMFCs), industrial (SOFC), stationary power (PAFC)
Advantages of Fuel Cells
- Environmental Advantages : No or minimal emission of pollutants
- Very Efficient : More efficient than combustion engines at converting fuel into electricity.
- Scalable: from portable devices to huge power plants
Fuel Cells : Challenges, Limitations and Future
Cost Factors
Fuel cells are costly owing to high cost of materials, especially catalysts eg, platinum.
Infrastructure Issues
The production of hydrogen is limited, as are the refueling stations.
Technical Challenges
Another major challenge is the long-term durability and performance degradation.
Advancements in Materials
Fuel cells need to become less expensive, so researchers are looking into more affordable and durable materials.
Potential Markets
Particularly for transportation, industrial applications, and residential power systems.
Importance in Integration of Renewable Energy
By storing & generating power from renewable resources, fuel cells can act as clean energy assets that augment our grid capacity.
Contact Lydia.sales1@tmnetch.com or visit our Facebook and LinkedIn page for further information.
How Do Hydrogen Cars Refuel and The Future of Fuel Cells in Vehicles
Five Ways in Which Coating Bipolar Plates can Improve Fuel Cell Efficiency
FAQs
Which kind of fuel cell is the most effective?
SOFCs are a very efficient type of fuel cell for stationary applications.
What are the types of fuel that can be utilized in fuel cells?
It runs primarily on hydrogen, but some types can be fueled with natural gas, biogas, or methanol.
Are fuel cells superior to batteries?
Fuel cells are more efficient than batteries for long duration and high power applications.
Is it possible for fuel cells to be used in vehicles?
Proton Exchange Membrane Fuel Cells (PEMFCs) are common in vehicles powered by hydrogen.
How long do fuel cells last?
Depending on the type, fuel cells have a working hours lifetime of 5,000 to more than 40,000 hours with maintenance.