What Are Proton Exchange Membranes?
A proton exchange membrane (PEM) acts as the central component that drives a fuel cell system operation. The polymer membrane functions as a thin substance that allows protons to transmit while acting as an obstruction to both electrons and gases. The partitioning mechanism plays an essential role in making fuel cells function for electrical production. As intermediaries, PEMs enable clean energy conversion to occur.
Importance of PEMs in Fuel Cell Technology and what is Perfluorosulfonic Acid?
PEM Technology enables the operation of electric vehicles together with portable electronics devices. The operation of hydrogen fuel cells depends completely on PEMs to function properly. PEMs play an essential role because they deliver high efficiency and compact size with near-zero emissions, which makes fuel cells stand out as the leading clean energy option of our time.
The Early Days of PEMs
Polystyrene Sulfonic Acid Membranes
Polystyrene sulfonic acid membranes served as the first choice for fuel cells at their inception. Proper laboratory tests indicated their strength, but their use in actual conditions proved ineffective. The membranes proved unstable when exposed to continuous operation and disappeared rapidly. The PEMs experienced quick failure, which led to their prompt abandonment.
Why They Failed
The main limitation of these membranes was that they did not have sufficient durability. Brand catalyzation led to a gradual deterioration of the material, which lost its conductivity capabilities and became rigid. The materials failed to endure both mechanical pressure and chemical reactions from working inside a fuel cell.
Nafion®: A Game-Changer
In the year 1960, DuPont introduced a PEM membrane based on perfluorosulfonic acid as its primary material. The membrane material named Nafion® showed improved characteristics for both proton conductivity and mechanical strength, together with excellent resistance to chemicals. PEM fuel cell technology adopted Nafion® as the premier standard for its operation.
What Makes Nafion® Special?
The backbone structure of Nafion® consists of Teflon dimensions, which leads to exceptional stability. Sulfonic acid groups on the side chain structures of Nafion® components allow the membrane to transport protons. The membrane achieves maximum durability through its chemical resistance along with excellent proton conduction properties.
The Functioning Mechanism of PFSA Membranes Inside Fuel Cells
During operation, the hydrogen at the anode splits with electrons moving separately from protons. The electrons move through an external circuit that produces electric power. The PFSA membrane allows proton movement from the anode toward the cathode until they reach the oxygen and electrons combination that forms water molecules.
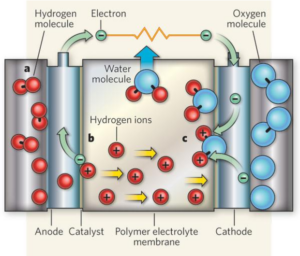
PFSA membranes function by letting protons move through their structure as they obstruct all other types of particles.
Benefits of PFSA Proton Exchange Membranes
Chemical and Thermal Stability
The PFSA membrane structure maintains its chemical structure when exposed to high acidity as well as warm operating temperatures. Pennsylvania State University and the University Trust can depend on PFSA membranes to operate reliably when exposed to severe environmental conditions.
High Proton Conductivity
The sulfonic acid groups in moist conditions create water-based proton passageways that allow rapid proton hopping across the membrane.
Mechanical Strength
The fluorinated backbone structure of PFSA provides superior mechanical strength to the membrane material. The material maintains integrity under continuous operations because it shows resistance to tearing and degradation.
Consistent Performance in Low-Temperature, High-Humidity Settings
The functioning capacity of PFSA membranes depends on sufficient moisture in conditions where temperature remains below specific threshold values.
Drawbacks and Limitations
Poor Performance at High Temperatures
The proton conductivity of PFSA membranes depends entirely on water content. Temperature increases pose a risk of membrane dryness, which leads to reduced efficiency, yet proper external humidification techniques are required to prevent this problem.
Water Management Is Tricky
The membrane loses its ability to conduct when it has insufficient water. The system becomes flooded when the water usage exceeds the optimal amount. The correct water concentration level demands continuous focus and attention to keep performance acceptable.
Methanol Permeability in DMFCs
PFSA membranes facilitate methanol flow between the DMFC anode and cathode compartments. When water crosses between compartments, it decreases performance levels while using up additional fuel.
High Production Costs
PFSA membranes have expensive production costs that stem from their labor-intensive production process as well as the high price of fluorinated polymers used in manufacturing.
Common Applications of PFSA Membranes
PEM Fuel Cells (PEMFCs)
PEM Fuel Cells together with stationary power units remain the most prevalent applications of these fuel cells. Efficiency and durability stand as critical advantages of PFSA membranes for their use in these applications.
Direct Methanol Fuel Cells (DMFCs)
The application of DMFCs serves portable electronic devices along with backup power systems. The use of PFSA membranes occurs frequently, although methanol crossover continues to be an obstructing factor.
Electrolyzers and Other Devices
PFSA membranes serve applications in electrolyzer hydrogen production together with several sensors and electrochemical devices, besides their primary role in fuel cells.
Emerging Alternatives to PFSA Membranes
Hydrocarbon-Based Membranes
The lower price of these membranes makes them suitable for high temperature operation, but they do not share the chemical resistance of PFSA membranes.
Composite Membranes
When PFSA incorporates silica or zirconia inorganic fillers, it results in better performance at elevated temperatures and reduces water loss.
Inorganic-Organic Hybrid Membranes
Scientists are working to create hybrid materials that unite PFSA benefits with other components to solve conductivity and cost problems.
Ongoing Research and Future Trends
Boosting High-Temperature Performance
Research teams work to create PFSA membranes that enhance water retention abilities to operate above temperatures exceeding 100°C, thus eliminating the requirement for external humidification.
Improving Durability and Longevity
Modern membrane manufacturing research aims to achieve extended life spans by remaining stable during stress situations. The outcome leads to membranes with higher resistance against both chemical destruction and physical deterioration.
Lowering Production Costs
The accessibility of PFSA membranes for commercial and residential energy solutions improves when manufacturing increases and when the project selects less expensive raw materials.
Conclusion
Proton exchange membranes made from perfluorosulfonic acid membranes have established themselves as a leading clean energy technology for multiple decades. PFSA membranes continue to be the most dependable solution for fuel cell applications due to their reliability, even though they require expensive production and experience some limitations with methanol passing through them. PFSA membranes are predicted to stay relevant and increase their leadership in the green energy sector because of active scientific developments.