In the actual process of building the new generation of efficient and environmentally friendly hydrogen technologies, PEM Water Electrolyzers play a special role. These electrolyzers are now at the forefront of the shift to more sustainable power sources with the increasing call for greener hydrogen. This article delves into the main components of PEM water electrolyzers and how they work to produce hydrogen through the electrolysis of water.
How PEM Electrolyzers Work
Basic Principle of Operation
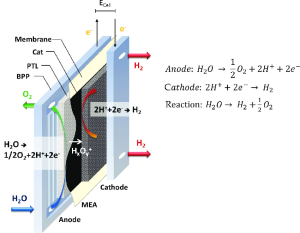
PEM electrolyzers use electrical energy to decompose water into hydrogen and oxygen; H₂O → H₂ + ½ O₂. It adopts electrolysis which involves passing an electric current through water and subsequently causing it to split into its relative fundamental physical constituents.
Process of Water Splitting
The electrochemical process of water splitting is well explained by immersing an electrode in a water solution where an electric current splits the water molecules into hydrogen ions or protons and oxygen. While in the process hydrogen ions move from one electrode to another through the PEM and oxygen is evolved at the anode. The protons then become attracted to the cathode where they join the electrons to form hydrogen Gas.
Main Components of PEM Water Electrolyzers
The PEM electrolyzers consist of several detailed parts as follows to facilitate the efficient process of electrolysis.
The Proton Exchange Membrane (PEM) : This is the core of the electrolyzer which allows the transport of protons but denies electrons. It usually includes materials like Nafion through which the transport of protons is facilitated.
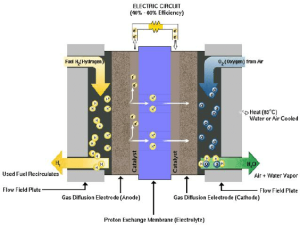
Electrodes: Electrodes are regarded as very essential in relation to the operation of the electrolysis process. An anode is where the oxygen evolves and a cathode is where hydrogen evolves.
Catalyst Layers : On the one hand, catalysts are employed to facilitate the extent of the exhibited reaction and to lower the energy required for water molecules to be split.
Proton Exchange Membrane Electrolyzer Parts
Membrane electrode assembly (MEA)
Basically, the MEA is an important part of PEM electrolyzers. It includes the deposition of a thin layer of the PEM in between two layers of the catalysts that are; the anode catalyst and the cathode catalyst.
Flow Field Plates
Electrolyzer component : Water and gases have to be guided through the electrolyzer by flow field plates. These contribute to the distribution of water across the electrodes and to controlling the gases evolve.

Electrolyzer Stack Design
The working and organization of stacks
To have a larger structure that can hold more electricity the stack of cells in the electrolyzer is an issue of design. Every cell in the array includes PEM, electrodes, and catalyst layers that are used in the production of Hydrogen.
Thermal Management
To speak more of the temperature characteristics, proper thermal management is important in order to maintain the operating temperature of the electrolyzer. High temperatures can slow down the efficiency of the system thus cooling processes are designed to be part of the stacks.
Materials Used in PEM Electrolyzers
The ease of performance and durability of PEM electrolyzers depend on the raw materials utilized in the construction of this equipment. Key materials include:
High-Conductivity Materials : For this purpose, this material such as platinum and iridium are incorporated in the intermediate cathode and anode layers to enable the electrolysis reaction.
Durability Considerations : The materials also cannot degrade over time due to conditions inside the electrolyzer, including high temperatures and chemical environments.
Catalyst Layers in PEM Electrolyzers
Function of Catalysts in Efficiency
Catalysts have been identified as crucial enablers of improved efficiency of PEM electrolyzers . This means the system only needs a smaller threshold amount of energy to activate water splitting since catalysts lower the amount of energy required.
Types of Catalysts
In the PEM electrolyzers, the preferred catalysts for the cathode are platinum, while for the anode, it is iridium.
It is found that materials used in the devices are highly efficient but costly and hence, the issue of making the technology a mass product is complicated.
Electrodes for PEM Electrolyzers
Anode and Cathode Design
In the anode configuration, oxygen is produced while in the cathode hydrogen is formed. In both electrode designs, an aggregate surface area must be provided to enhance the efficiency of the electrolysis reaction.
Materials Used for Electrodes
Platinum and iridium are used often for the electrode catalyst layers, and titanium is used for the structures of the electrodes.
PEM Electrolyzer Membrane Material
Characteristics of PEM
As a potential ion conductor, the PEM is designed to allow the passage of protons while blocking such gaseous species as hydrogen and oxygen. This is so important for the preservation of the system and to ensure that electrolysis operation is accomplished properly.
Role in Proton Transport
The PEM has a significant function of facilitating proton transport through the membrane and from the anode to the cathode where the protons combine with the electrons to give hydrogen gas.
How to Improve PEM Electrolyzer Efficiency
Improving the efficiency of PEM electrolyzers involves optimizing several key components:
Improving Catalyst Performance: Enhancing the catalysts will go a long way in decreasing
the energy needed for the electrolysis process.
Enhancing Membrane Durability: It appears that the PEM needs to be very long-lived in order to achieve good long-term performance. But the attempts are being made by researchers in materials science to design and fabricate membranes that can effectively work under stressed conditions.
Water Splitting in PEM Electrolyzers
Optimization of Electrolysis: Electrolysis Process
The electrolysis process also refers to passing an electric current through water so as to decompose the water into hydrogen and oxygen. The PEM makes sure Protons move in an optimal manner while at the same time denying gases a chance to escape the system.
Conversion Of Water To Hydrogen Efficiency
The hydrogen recovery rate for extraction from water depends on the performance of the PEM , catalysts, and overall construction of the electrolyzer.
Recent Advancements in Technology
Recent updates in PEM electrolyzers are pointing to improvements in the efficiency of the catalysts used in the electrolyzers and enhancement in stack designs that also contribute towards cutting the costs of PEM electrolyzers.
PEM Electrolyzers usage
Opportunities for clean hydrogen technologies are needed to make a significant contribution to cleaning up the power sector which at the moment is dominated by the use of fossil fuels.
The PEM electrolyzers are crucial in the generation of green hydrogen which is hydrogen generated from renewable energy input. This puts them in the driver’s seat of transition to a sustainable energy system.
It is widely used in Renewable Energy Systems.
Currently, PEM electrolyzers are incorporated into renewable power systems for generating hydrogen that may be stored as a clean fuel.
The PEM water electrolyzers of the future
Potential for Mass Deployment
Because PEM electrolyzers are already advanced and are becoming cheaper by the day, they can be used at a large scale for the purpose of producing hydrogen.
Current Research and developments
A current area of active research is focused on optimizing the efficiency, cost, and longevity of PEM electrolyzers, which makes the technology all the more attractive for future green energy.
PEM water electrolyzers are one of the vital technologies sought after in the quest for green hydrogen and sustainable energy solutions. When we realize the parts and how they interact, is when we get the big picture of how difficult the process of making PEM electrolyzers efficient and affordable is, and how great the efforts of innovation are being put into making it so. The generation of clean hydrogen in the future seems to be bright, especially with the accompanying improvement of PEM electrolyzers .
Visit our LinkedIn page for further details.
Exploring PEM Fuel Cells: Working, Efficiency, Applications, and Future
FAQs About PEM Electrolyzers
What is the role of the proton exchange membrane (PEM) in an electrolyzer?
The PEM allows protons to move from the anode to the cathode while preventing gases like hydrogen and oxygen from mixing.
How efficient are PEM electrolyzers in producing hydrogen?
PEM electrolyzers are highly efficient, with some systems achieving efficiencies of up to 70-80% in converting electricity into hydrogen.
What materials are used in the catalysts for PEM electrolyzers?
The most commonly used materials for catalysts are platinum and iridium, which are highly efficient at facilitating the electrolysis reaction.
Can PEM electrolyzers be used with renewable energy sources?
Yes, PEM electrolyzers can be powered by renewable energy sources like solar and wind to produce green hydrogen.
What are the challenges facing PEM electrolyzers ?
The main challenges include high costs due to the use of expensive materials and the need for better durability and efficiency.

